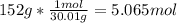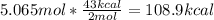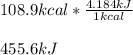Chemistry, 11.02.2021 14:00 santiagoagilg
One mole (mol) of nitrogen monoxide (NO) has a mass of 30.01 g. When
precisely 2 moles of NO(g) are produced in the following chemical reaction, 43
kcal of heat energy is "absorbed."
N2(g) + O2(g) → 2 NO(g), AH = +43 kcal
How much heat (in kJ) is exchanged when 152 g of NO(g) is produced?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
You know the right answer?
One mole (mol) of nitrogen monoxide (NO) has a mass of 30.01 g. When
precisely 2 moles of NO(g) are...
Questions


Biology, 04.08.2019 11:00

Social Studies, 04.08.2019 11:00

History, 04.08.2019 11:00

Social Studies, 04.08.2019 11:00

Chemistry, 04.08.2019 11:00

Biology, 04.08.2019 11:00

Social Studies, 04.08.2019 11:00

Biology, 04.08.2019 11:00

Business, 04.08.2019 11:00

Biology, 04.08.2019 11:00

Biology, 04.08.2019 11:00


Chemistry, 04.08.2019 11:00

History, 04.08.2019 11:00

Biology, 04.08.2019 11:00

Mathematics, 04.08.2019 11:00