Chemistry, 11.02.2021 17:40 mshepherdmiller
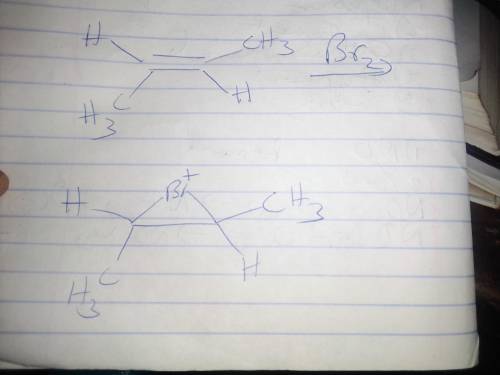
Draw the bridged bromonium ion that is formed as an intermediate during the bromination of this alkene. Include hydrogen atoms, nonbonding electrons, and formal charge(s) in your structure. The starting alkene is a 4 carbon chain with a double bond between carbons 2 and 3. The substituents on the alkene are on opposite sides of the alkene. This reacts with B r 2 to give the intermediate ion.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Draw the bridged bromonium ion that is formed as an intermediate during the bromination of this alke...
Questions

Mathematics, 28.05.2021 23:20

Advanced Placement (AP), 28.05.2021 23:20




Mathematics, 28.05.2021 23:20


Mathematics, 28.05.2021 23:20

Mathematics, 28.05.2021 23:20


Mathematics, 28.05.2021 23:20

Computers and Technology, 28.05.2021 23:20




Computers and Technology, 28.05.2021 23:20

Health, 28.05.2021 23:20



Mathematics, 28.05.2021 23:20