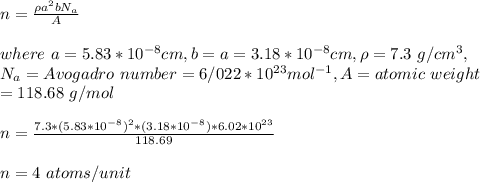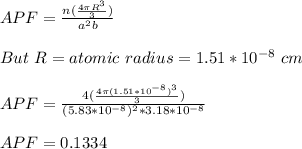Chemistry, 11.02.2021 21:10 yejinschoi6362
The unit cell for tin (Sn) has tetragonal symmetry, with a and b lattice parameters of 0.583 and 0.318 nm, respectively. If its density, atomic weight, and atomic radius are 7.30 g/cm3, 118.69 g/mol, and 0.151 nm, respectively. Determine its atomic packing factor.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3
You know the right answer?
The unit cell for tin (Sn) has tetragonal symmetry, with a and b lattice parameters of 0.583 and 0.3...
Questions



English, 22.07.2021 08:40

English, 22.07.2021 08:40

Mathematics, 22.07.2021 08:40

Mathematics, 22.07.2021 08:40

Mathematics, 22.07.2021 08:40


Mathematics, 22.07.2021 08:40

Mathematics, 22.07.2021 08:40



Social Studies, 22.07.2021 08:40

German, 22.07.2021 08:40

World Languages, 22.07.2021 08:40

Mathematics, 22.07.2021 08:40

Social Studies, 22.07.2021 08:40


English, 22.07.2021 08:40

Computers and Technology, 22.07.2021 08:40