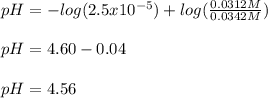Use the Henderson-Hasselbalch equation, eq. (3), to calculate the pH expected for a buffer solution prepared from this acid and its conjugate base, assuming: the mass of the weak acid is 0.2 g and the mass of the conjugate base is 0.2 g will be added to 25 mL of water. Also assume the molar mass of the weak acid is 234 and its conjugate base is the sodium salt.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Use the Henderson-Hasselbalch equation, eq. (3), to calculate the pH expected for a buffer solution...
Questions

Social Studies, 30.08.2020 08:01

Biology, 30.08.2020 08:01

English, 30.08.2020 08:01

Mathematics, 30.08.2020 08:01

Mathematics, 30.08.2020 08:01

Advanced Placement (AP), 30.08.2020 08:01


Mathematics, 30.08.2020 08:01


Geography, 30.08.2020 08:01

English, 30.08.2020 08:01




English, 30.08.2020 08:01

Mathematics, 30.08.2020 08:01

Physics, 30.08.2020 08:01


Advanced Placement (AP), 30.08.2020 08:01

Social Studies, 30.08.2020 08:01

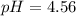
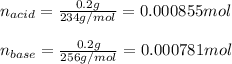
![[acid]=0.000855mol/0.025L=0.0342M](/tpl/images/1113/0020/c5975.png)
![[base]=0.000781mol/0.025L=0.0312M](/tpl/images/1113/0020/790f7.png)