
Chemistry, 12.02.2021 05:20 nancye2008
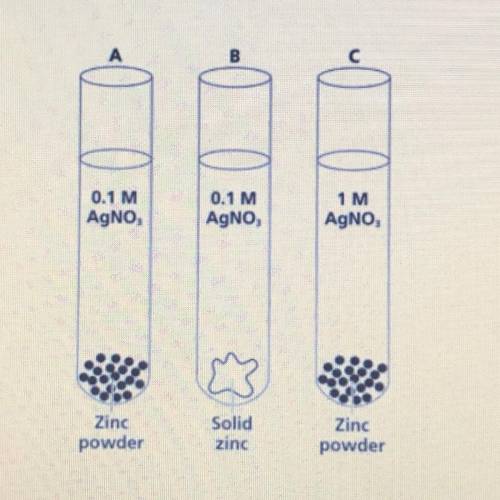
Which of the following test tubes
A) would have the fastest rate of reaction? Defend your answer.
B) would have the slowest rate of reaction? Defend your answer.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Construct the hypothetical phase diagram for metals a and b between room temperature (20c) and 700c, given the following information: * the melting temperature of metal a is 480c. • the maximum solubility of b in a is 4 wt% b, which occurs at 420c. • the solubility of b in a at room temperature is 0 wt% b. • one eutectic occurs at 420c and 18 wt% b–82 wt% a. • a second eutectic occurs at 475c and 42 wt% b–58 wt% a. • the intermetallic compound ab exists at a composition of 30 wt% b–70 wt% a, and melts congruently at 525c.• the melting temperature of metal b is 600c. • the maximum solubility of a in b is 13 wt% a, which occurs at 475c. • the solubility of a in b at room temperature is 3 wt% a.
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

You know the right answer?
Which of the following test tubes
A) would have the fastest rate of reaction? Defend your answer.
Questions



Mathematics, 16.12.2020 14:30


English, 16.12.2020 14:30

History, 16.12.2020 14:30

Health, 16.12.2020 14:30

Physics, 16.12.2020 14:30


English, 16.12.2020 14:30



Mathematics, 16.12.2020 14:30

Spanish, 16.12.2020 14:30

Chemistry, 16.12.2020 14:30


Biology, 16.12.2020 14:30

Arts, 16.12.2020 14:30

Mathematics, 16.12.2020 14:30



