
Chemistry, 19.09.2019 18:30 woodfordmaliky
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(aq)+3h2(g) (deltah)= -725.0 kj
2. hcl(> hcl(aq) (deltah)= -74.8kj 3. h2(g)+cl2(g) --> 2hcl(g) (deltah)=-1845.0kj 4. mcl3(s) --> mcl3(aq) (deltah)= -476.0kj use the information above to determine the enthalpy of the following reaction.
2m(s)+3cl2(g) > 2mcl3(s) (deltah) = kj

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 14:00
Which word refers to the smallest functional unit of living thing
Answers: 1


Chemistry, 23.06.2019 16:00
Why is it important for scientists to replicate each other’s experiments? to determine if important scientific results are repeatable to the research of other scientists to determine if slight alterations in the experiment can affect the result to further their own research
Answers: 2
You know the right answer?
Consider these reactions where m represents a generic metal
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
1. 2m(s) +6hcl(aq) --> 2mcl3(a...
Questions

Chemistry, 24.03.2020 19:57










Mathematics, 24.03.2020 19:57



Mathematics, 24.03.2020 19:57


Mathematics, 24.03.2020 19:57

Biology, 24.03.2020 19:57



Mathematics, 24.03.2020 19:57

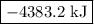
 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.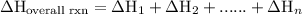
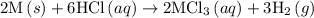 ...… (1)
...… (1)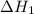 is -725 kJ.
is -725 kJ.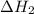 .
.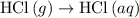 ...... (2)
...... (2) 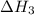 .
. …… (3)
…… (3)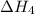 .
.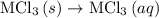 …… (4)
…… (4)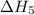 .
. …… (5)
…… (5)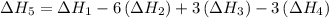 ...... (7)
...... (7) 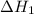 , -74.8 kJ for
, -74.8 kJ for 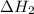 and -1845 kJ for
and -1845 kJ for 
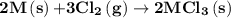 is -4383.2 kJ.
is -4383.2 kJ.

