
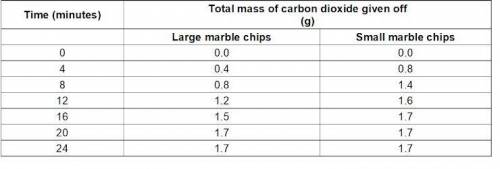
A student investigates the rate of reaction between marble chips and hydrochloric acid. Both experiments use 50 cm3 of hydrochloric acid and an excess of marble chips. He measures the total mass of carbon dioxide given off for different sizes of marble chips. Look at his results. The student has plotted his results for the large marble chips on the graph (in section below) . What would the line for small marble chips look like?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3


Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
A student investigates the rate of reaction between marble chips and hydrochloric acid. Both experim...
Questions

English, 01.07.2019 14:00

Geography, 01.07.2019 14:00

Spanish, 01.07.2019 14:00



Mathematics, 01.07.2019 14:00


English, 01.07.2019 14:00

Business, 01.07.2019 14:00


Chemistry, 01.07.2019 14:00




Physics, 01.07.2019 14:00

Mathematics, 01.07.2019 14:00

Social Studies, 01.07.2019 14:00



Physics, 01.07.2019 14:00



