
At 298 K, the Henry's law constant for oxygen is 0.00130 M/atm. Air is 21.0% oxygen.
1) At 298 K, what is the solubility of oxygen in water exposed to air at 1.00 atm?
2) At 298 K, what is the solubility of oxygen in water exposed to air at 0.89 atm?
3) If atmospheric pressure suddenly changes from 1.00 atm to 0.893 atm at 298 K, how much oxygen will be released from 4.70L of water in an unsealed container?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1
You know the right answer?
At 298 K, the Henry's law constant for oxygen is 0.00130 M/atm. Air is 21.0% oxygen.
1) At 298 K, w...
Questions


Mathematics, 04.03.2020 02:12







Mathematics, 04.03.2020 02:13

Advanced Placement (AP), 04.03.2020 02:13

Mathematics, 04.03.2020 02:13


English, 04.03.2020 02:13



Mathematics, 04.03.2020 02:13

Social Studies, 04.03.2020 02:14

History, 04.03.2020 02:14

Geography, 04.03.2020 02:14

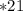 % of atm O2
% of atm O2 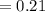 atm
atm
 mole / L
mole / L
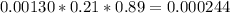 mole / L
mole / L
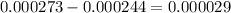
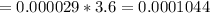 mole
mole 


