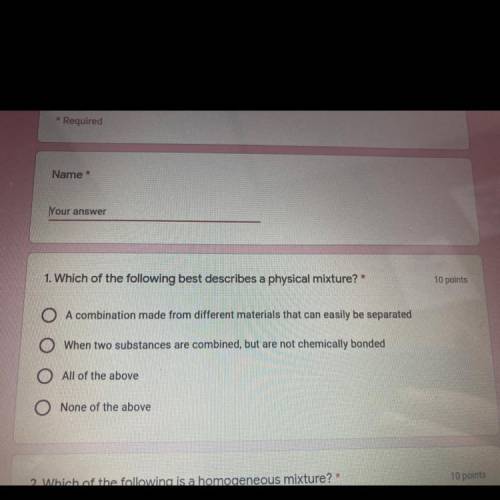
I need help please please
...

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3

Chemistry, 23.06.2019 21:30
Acompound containing nitrogen and oxygen is decomposed in the laboratory and produces 1.78 g of nitrogen and 4.05 g of oxygen. part a calculate the empirical formula of the compound.
Answers: 1
You know the right answer?
Questions





History, 30.10.2020 16:30






History, 30.10.2020 16:30


Geography, 30.10.2020 16:30


Mathematics, 30.10.2020 16:30

Mathematics, 30.10.2020 16:30



English, 30.10.2020 16:30

Mathematics, 30.10.2020 16:30