Name
Virtual Chemistry
Unit 7 HW 4
Answer questions 1-12 about the phase diagram shown...

Chemistry, 12.02.2021 18:30 gabriellabadon2
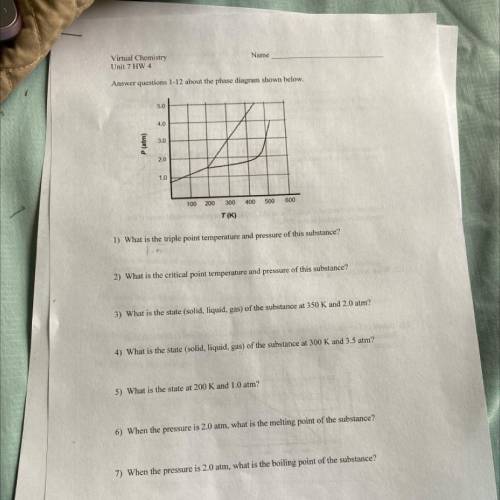
Name
Virtual Chemistry
Unit 7 HW 4
Answer questions 1-12 about the phase diagram shown below.
5.0
4.0
3.0
P (atm)
2.0
1.0
100 200
300
400
500
600
T(K)
1) What is the triple point temperature and pressure of this substance?
2) What is the critical point temperature and pressure of this substance?
3) What is the state (solid, liquid, gas) of the substance at 350 K and 2.0 atm?
4) What is the state (solid, liquid, gas) of the substance at 300 K and 3.5 atm?
5) What is the state at 200 K and 1.0 atm?
6) When the pressure is 2.0 atm, what is the melting point of the substance?
7) When the pressure is 2.0 atm, what is the boiling point of the substance?

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
Questions

Chemistry, 06.11.2020 14:00


Mathematics, 06.11.2020 14:00





Mathematics, 06.11.2020 14:00

Mathematics, 06.11.2020 14:00




Business, 06.11.2020 14:00

Mathematics, 06.11.2020 14:00

Physics, 06.11.2020 14:00


Computers and Technology, 06.11.2020 14:00



Arts, 06.11.2020 14:00



