Chemistry, 12.02.2021 21:10 kiaraahquin9546
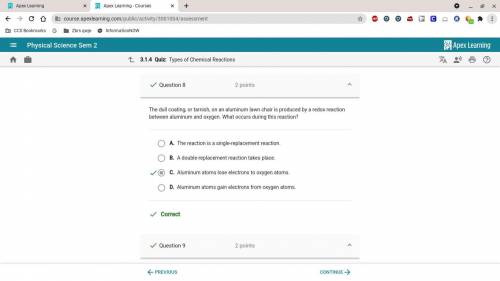
The dull coating, or tarnish, on an aluminum lawn chair is produced by a
redox reaction between aluminum and oxygen. What occurs during this
reaction?
A. A double-replacement reaction takes place.
B. The reaction is a single-replacement reaction.
O C. Aluminum atoms lose electrons to oxygen atoms.
D. Aluminum atoms gain electrons from oxygen atoms.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 12:30
Given 7.65 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield?
Answers: 3

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
The dull coating, or tarnish, on an aluminum lawn chair is produced by a
redox reaction between alu...
Questions

Biology, 30.08.2021 21:00

Mathematics, 30.08.2021 21:00

Mathematics, 30.08.2021 21:00


Mathematics, 30.08.2021 21:00

History, 30.08.2021 21:00

Mathematics, 30.08.2021 21:00





History, 30.08.2021 21:00


History, 30.08.2021 21:00

Mathematics, 30.08.2021 21:00

History, 30.08.2021 21:00

Mathematics, 30.08.2021 21:00



Mathematics, 30.08.2021 21:00