
Chemistry, 14.02.2021 01:20 NateTheBeast12
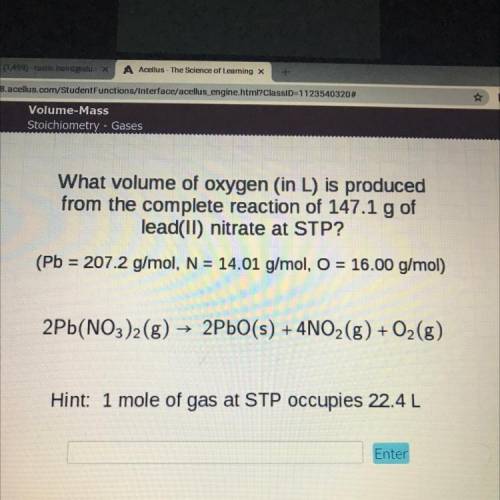
What volume of oxygen (in L) is produced
from the complete reaction of 147.1 g of
lead(II) nitrate at STP?
(Pb = 207.2 g/mol, N = 14.01 g/mol, O = 16.00 g/mol)
2Pb(NO3)2(g) → 2PbO(s) + 4NO2(g) + O2(g)
Hint: 1 mole of gas at STP occupies 22.4L

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2
You know the right answer?
What volume of oxygen (in L) is produced
from the complete reaction of 147.1 g of
lead(II) ni...
lead(II) ni...
Questions






History, 15.04.2021 20:10


Mathematics, 15.04.2021 20:10

English, 15.04.2021 20:10

Mathematics, 15.04.2021 20:10


Mathematics, 15.04.2021 20:10


Mathematics, 15.04.2021 20:10


English, 15.04.2021 20:10


History, 15.04.2021 20:10


Mathematics, 15.04.2021 20:10



