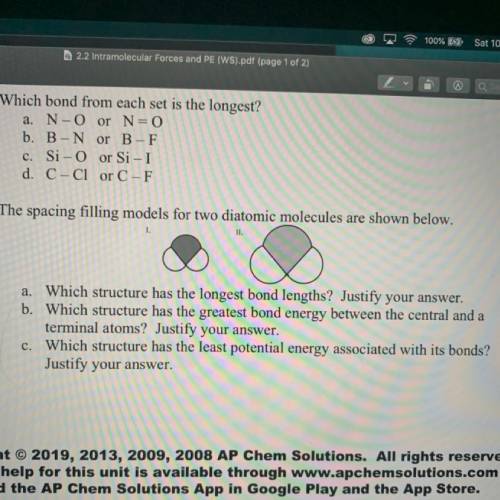
5) The spacing filling models for two diatomic molecules are shown below.
1.
II.
a. Whi...

Chemistry, 14.02.2021 06:20 Emilybaez15
5) The spacing filling models for two diatomic molecules are shown below.
1.
II.
a. Which structure has the longest bond lengths? Justify your answer.
b. Which structure has the greatest bond energy between the central and a
terminal atoms? Justify your answer.
c. Which structure has the least potential energy associated with its bonds?
Justify your answer.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
Questions

Geography, 06.07.2019 18:00

Social Studies, 06.07.2019 18:00



Biology, 06.07.2019 18:00

Chemistry, 06.07.2019 18:00

Chemistry, 06.07.2019 18:00

Biology, 06.07.2019 18:00


English, 06.07.2019 18:00



Chemistry, 06.07.2019 18:00



Physics, 06.07.2019 18:00

History, 06.07.2019 18:00


Mathematics, 06.07.2019 18:00

Mathematics, 06.07.2019 18:00



