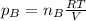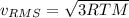Chemistry, 15.02.2021 19:40 mikehager4321
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Which of the two gases exerts the greater partial pressure?
b. The molecules or atoms of which gas have the greater average velocity?
c. The molecules of which gas have the greater average kinetic energy?
d . If a small hole were opened in the flask, which gas effuses more quickly?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1


Chemistry, 23.06.2019 10:30
Which of the following characteristics are true of enzymes? check all that apply. a.)the structure of an enzyme can change if conditions change. b.)a single enzyme can normally catalyze a wide variety of reactions under many conditions. c.)enzymes are found only in nonliving systems. d.)enzymes allow living things to regulate body conditions through feedback mechanisms. e.)enzymes bind to specific substrates in specific ways. f.)enzymes increase the rate of reaction. g.)when shown in energy-reaction diagrams, enzymes represent the higher activation energy.
Answers: 1
You know the right answer?
A flask at room temperature contains exactly equal amounts (in moles) of nitrogen and xenon.
a. Whi...
Questions

Mathematics, 03.03.2021 19:50

Geography, 03.03.2021 19:50


Mathematics, 03.03.2021 19:50

Mathematics, 03.03.2021 19:50


Mathematics, 03.03.2021 19:50



History, 03.03.2021 19:50

English, 03.03.2021 19:50



Mathematics, 03.03.2021 19:50

Mathematics, 03.03.2021 19:50

Mathematics, 03.03.2021 19:50



Arts, 03.03.2021 19:50

Mathematics, 03.03.2021 19:50