
Chemistry, 15.02.2021 21:10 kieramacphee6216
Estimate how much heat in joules is released when 25.0 g of water (C = 4.184 J/g°C) is cooled from 80.0°C to 30.0°C?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1

Chemistry, 23.06.2019 09:00
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2
You know the right answer?
Estimate how much heat in joules is released when 25.0 g of water (C = 4.184 J/g°C) is cooled from 8...
Questions

English, 27.01.2021 15:30




Mathematics, 27.01.2021 15:30

History, 27.01.2021 15:30



Chemistry, 27.01.2021 15:30

Physics, 27.01.2021 15:30


Mathematics, 27.01.2021 15:30

Chemistry, 27.01.2021 15:30

Spanish, 27.01.2021 15:30


Social Studies, 27.01.2021 15:30

Mathematics, 27.01.2021 15:30



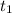 = 80 °C
= 80 °C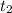 = 30 °C
= 30 °C )
) 


