
Chemistry, 15.02.2021 22:10 marandahuber
N2+3H2=>>2NH3. If 6.72dm³ of each of Nitrogen and hydrogen (measured at S. T.P.) are made to produced Ammonium. Which of the reacted will be in excess and by how many moles?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2
You know the right answer?
N2+3H2=>>2NH3. If 6.72dm³ of each of Nitrogen and hydrogen (measured at S. T.P.) are made to p...
Questions







Computers and Technology, 13.03.2020 22:38

Mathematics, 13.03.2020 22:38


Computers and Technology, 13.03.2020 22:38



Chemistry, 13.03.2020 22:38







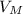 = 22, 4 l/mol
= 22, 4 l/mol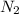 ) = 6,72
) = 6,72 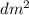 = 6,72 l
= 6,72 l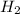 ) = 6,72
) = 6,72 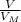
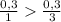 (we have more hydrogen than is need for the reaction)
(we have more hydrogen than is need for the reaction)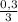 = 0,1 mol (
= 0,1 mol (

