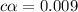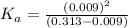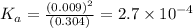Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3


Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
In the laboratory, a general chemistry student measured the pH of a 0.313 M aqueous solution of acet...
Questions

Mathematics, 14.12.2020 01:50




SAT, 14.12.2020 01:50



Mathematics, 14.12.2020 01:50


Chemistry, 14.12.2020 01:50

Mathematics, 14.12.2020 01:50


Mathematics, 14.12.2020 01:50


Biology, 14.12.2020 01:50

Social Studies, 14.12.2020 01:50


Mathematics, 14.12.2020 01:50


Computers and Technology, 14.12.2020 01:50

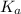 for the acid is
for the acid is 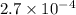
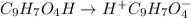
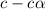
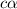
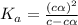
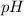 = 2.031
= 2.031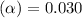
![[H^+]=c\times \alpha](/tpl/images/1121/0617/4fc41.png)
![[H^+]=0.500\times 0.030=0.015](/tpl/images/1121/0617/28636.png)
![pH=-log[H^+]](/tpl/images/1121/0617/15713.png)
![2.031=-log[H^+]](/tpl/images/1121/0617/0b1a6.png)
![[H^+]=0.009](/tpl/images/1121/0617/13241.png)
![[H^+]=c\alpha](/tpl/images/1121/0617/21a04.png)