
Chemistry, 16.02.2021 04:20 baileyflemingde
Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How many grams of sodium azide are required to produce 24.4 L of nitrogen gas at standard temperature and pressure? 2NaN3 --> 2Na + 3N2
47.2 g of sodium azide
106.2 g of sodium azide
1.63 g of sodium azide
0.726 g of sodium azide

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3
You know the right answer?
Automotive airbags inflate when sodium azide decomposes explosively to its constituent elements. How...
Questions

Mathematics, 19.07.2020 14:01

Chemistry, 19.07.2020 14:01

Engineering, 19.07.2020 14:01


Mathematics, 19.07.2020 14:01

Mathematics, 19.07.2020 14:01


Physics, 19.07.2020 14:01

English, 19.07.2020 14:01

Mathematics, 19.07.2020 14:01

Mathematics, 19.07.2020 14:01

Biology, 19.07.2020 14:01

Mathematics, 19.07.2020 14:01







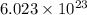 of particles.
of particles.


 are produced by = 2 moles of
are produced by = 2 moles of 
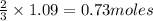 of
of 



