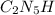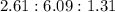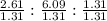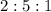Chemistry, 16.02.2021 06:00 BossKnight
After the shuttle disaster, an unknown compound residue was removed from a piece of the debris. Upon analysis, it was found to contain 2.61 g of carbon, 6.09 g of nitrogen, and 1.31 g of hydrogen. What is its empirical formula?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

You know the right answer?
After the shuttle disaster, an unknown compound residue was removed from a piece of the debris. Upon...
Questions

Mathematics, 22.04.2020 18:01

Mathematics, 22.04.2020 18:01

History, 22.04.2020 18:01




Biology, 22.04.2020 18:01




Geography, 22.04.2020 18:01

Geography, 22.04.2020 18:01

Mathematics, 22.04.2020 18:01


Mathematics, 22.04.2020 18:01

History, 22.04.2020 18:01



Mathematics, 22.04.2020 18:01