
Chemistry, 16.02.2021 07:10 crystaldewar55C
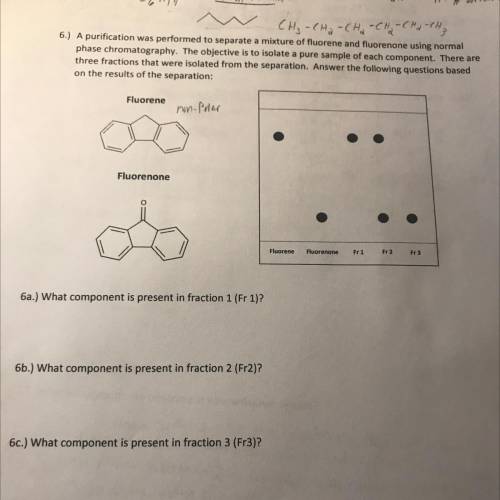
6.) A purification was performed to separate a mixture of fluorene and fluorenone using normal
phase chromatography. The objective is to isolate a pure sample of each component. There are
three fractions that were isolated from the separation. Answer the following questions based
on the results of the separation:
Fluorene
non-Paar
Fluorenone
Fluorene
Fluorenone
Fr 1
Fr 2
Fr3
6a.) What component is present in fraction 1 (Fr 1)?
6b.) What component is present in fraction 2 (Fr2)?
6c.) What component is present in fraction 3 (Fr3)?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
6.) A purification was performed to separate a mixture of fluorene and fluorenone using normal
phas...
Questions


Social Studies, 06.03.2022 08:10

Mathematics, 06.03.2022 08:10

Health, 06.03.2022 08:10


Biology, 06.03.2022 08:10

Spanish, 06.03.2022 08:10



Physics, 06.03.2022 08:20


English, 06.03.2022 08:20










