
Chemistry, 16.02.2021 23:40 josephmelichar777
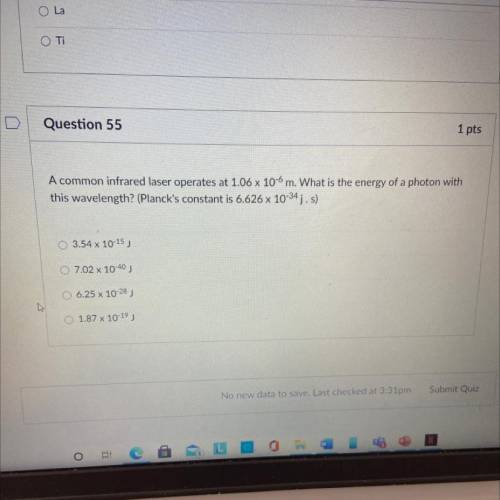
A common infrared laser operates at 1.06 x 100 m. What is the energy of a photon with this wavelength? (Planck's constant is 6.626 x 10-34 j. s)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

You know the right answer?
A common infrared laser operates at 1.06 x 100 m. What is the energy of a photon with
this waveleng...
Questions

History, 21.08.2020 17:01



Mathematics, 21.08.2020 17:01

Chemistry, 21.08.2020 17:01

English, 21.08.2020 17:01


Computers and Technology, 21.08.2020 17:01



Mathematics, 21.08.2020 17:01

Mathematics, 21.08.2020 17:01


Mathematics, 21.08.2020 17:01

Biology, 21.08.2020 17:01

History, 21.08.2020 17:01



Social Studies, 21.08.2020 17:01



