
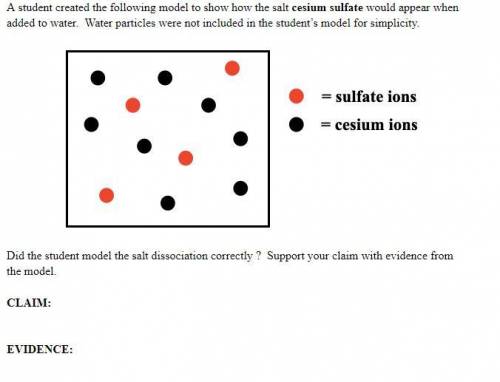
A student created the following model to show how the salt cesium sulfate would appear when added to water. Water particles were not included in the student’s model for simplicity. Did the student model the salt dissociation correctly ? Support your claim with evidence from the model.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
A student created the following model to show how the salt cesium sulfate would appear when added to...
Questions











Mathematics, 21.09.2019 04:30

English, 21.09.2019 04:30


Chemistry, 21.09.2019 04:30


English, 21.09.2019 04:30







