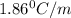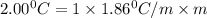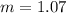Chemistry, 17.02.2021 09:20 milkshakegrande101
What is the molal concentration of a sucrose (C12H22011) solution whose freezing point is -2.00 °C? The Kf for water is 1.86 °C/m.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
What is the molal concentration of a sucrose (C12H22011) solution whose freezing point is
-2.00 °C?...
Questions

Mathematics, 25.10.2021 14:00


History, 25.10.2021 14:00

World Languages, 25.10.2021 14:00

Social Studies, 25.10.2021 14:00


Social Studies, 25.10.2021 14:00

Business, 25.10.2021 14:00

English, 25.10.2021 14:00


History, 25.10.2021 14:00



History, 25.10.2021 14:00

World Languages, 25.10.2021 14:00

Mathematics, 25.10.2021 14:00



History, 25.10.2021 14:00

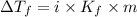
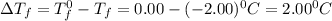 = Depression in freezing point
= Depression in freezing point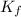 = freezing point constant =
= freezing point constant =