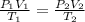Chemistry, 17.02.2021 14:10 jfitness11
A gas in a piston starts out with a volume of 156 mL, a temperature of 28.1° C, and a pressure of 1.12 atm. If it ends with a volume of 312 mL and a temperature of 87.2° C, what is the new pressure?


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1


Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 08:40
The half-life of a certain element is 100 days. how many half-lives will it be before only one eighth of this elementremains?
Answers: 1
You know the right answer?
A gas in a piston starts out with a volume of 156 mL, a temperature of 28.1° C, and a pressure of 1....
Questions

World Languages, 10.01.2021 05:00

Mathematics, 10.01.2021 05:00

Social Studies, 10.01.2021 05:00

Mathematics, 10.01.2021 05:00


Mathematics, 10.01.2021 05:00

Mathematics, 10.01.2021 05:00

Mathematics, 10.01.2021 05:00


Mathematics, 10.01.2021 05:00


Mathematics, 10.01.2021 05:00

Biology, 10.01.2021 05:00


Mathematics, 10.01.2021 05:00



Computers and Technology, 10.01.2021 05:00

History, 10.01.2021 05:00

History, 10.01.2021 05:00