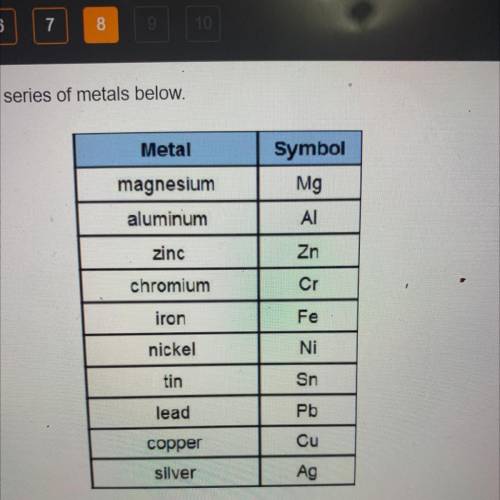
The following reaction occurs in an electrochemical cell.
Sn2++ Pb → Pb2++ Sn
What type of el...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Questions

History, 02.11.2019 08:31




Biology, 02.11.2019 08:31






Mathematics, 02.11.2019 08:31


Mathematics, 02.11.2019 08:31

Biology, 02.11.2019 08:31

English, 02.11.2019 08:31


Mathematics, 02.11.2019 08:31



Mathematics, 02.11.2019 08:31