Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
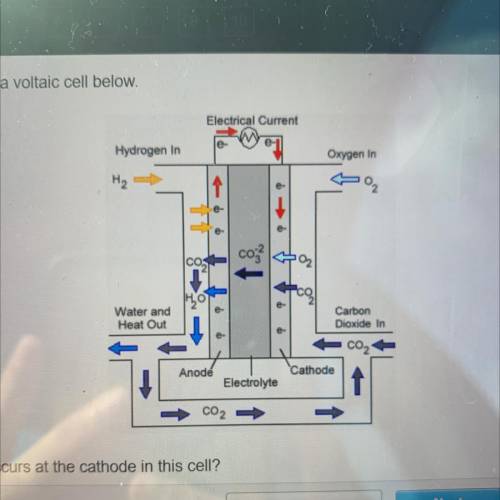
Which half reaction occurs at the cathode in this cell?
2003--O2 + 2002 + 4e-
+ 2002 + 4e--&g...
+ 2002 + 4e--&g...
Questions


History, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40

Arts, 18.11.2020 22:40


Mathematics, 18.11.2020 22:40




Mathematics, 18.11.2020 22:40



Spanish, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40


Biology, 18.11.2020 22:40

Mathematics, 18.11.2020 22:40