
Chemistry, 18.02.2021 01:00 burritomadness
How do I make the molar masses for 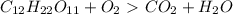 (reactant and products) equal? Please, tell me the steps and how to recalculate.
(reactant and products) equal? Please, tell me the steps and how to recalculate.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
How do I make the molar masses for (reactant and products) equal? Please, tell me the steps and how...
Questions

History, 16.12.2020 01:50

Mathematics, 16.12.2020 01:50

History, 16.12.2020 01:50



History, 16.12.2020 01:50




Advanced Placement (AP), 16.12.2020 01:50

Mathematics, 16.12.2020 01:50

Chemistry, 16.12.2020 01:50

Mathematics, 16.12.2020 01:50

Mathematics, 16.12.2020 01:50




Computers and Technology, 16.12.2020 01:50




