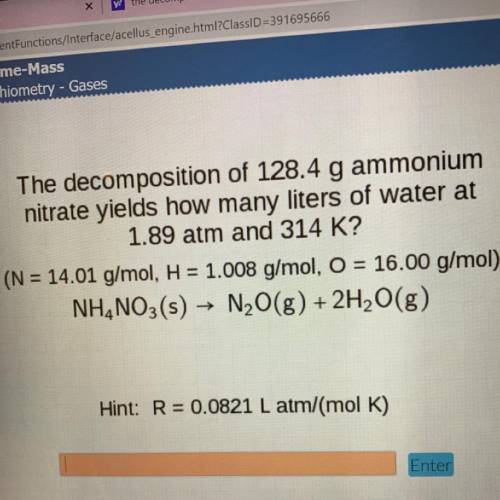
The decomposition of 128.4 g ammonium
nitrate yields how many liters of water at
1.89 atm and...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2

Chemistry, 23.06.2019 07:40
Which of the following has expanded our knowledge of the universe beyond our solar system the most? a. manned space travel b. the hubble space telescope c. the pioneer and voyager missions d. the international space station
Answers: 3
You know the right answer?
Questions

Mathematics, 04.08.2019 05:30

English, 04.08.2019 05:30

History, 04.08.2019 05:30

Mathematics, 04.08.2019 05:30

Mathematics, 04.08.2019 05:30

Mathematics, 04.08.2019 05:30

Mathematics, 04.08.2019 05:30












Mathematics, 04.08.2019 05:30