
Chemistry, 18.02.2021 09:20 kaylamount
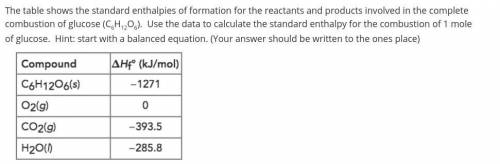
The table shows the standard enthalpies of formation for the reactants and products involved in the complete combustion of glucose (C6H12O6). Use the data to calculate the standard enthalpy for the combustion of 1 mole of glucose. Hint: start with a balanced equation. (Your answer should be written to the ones place)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

You know the right answer?
The table shows the standard enthalpies of formation for the reactants and products involved in the...
Questions


Business, 12.03.2020 20:21




Mathematics, 12.03.2020 20:21




History, 12.03.2020 20:21


Computers and Technology, 12.03.2020 20:21



Mathematics, 12.03.2020 20:21



Mathematics, 12.03.2020 20:22




