
Chemistry, 18.02.2021 21:00 larissa1013
suppose you start out with only reactants in a rigid container. if the initial concentration of SO2Cl2(g) is 0.543 M, and 43.6% of this initial concentration remains when the system has reached equilibrium, what are the equilibrium concentrations of each gas in the system

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3
You know the right answer?
suppose you start out with only reactants in a rigid container. if the initial concentration of SO2C...
Questions


English, 29.01.2020 04:57


English, 29.01.2020 04:57

Social Studies, 29.01.2020 04:57



English, 29.01.2020 04:57


Mathematics, 29.01.2020 04:57



History, 29.01.2020 04:57




SAT, 29.01.2020 04:57


Geography, 29.01.2020 04:57

Geography, 29.01.2020 04:57

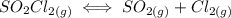
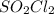 that is being reacted is:
that is being reacted is: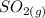 +
+ 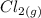
![K_c = \dfrac{[SO_2] [Cl_{2}]}{[SO_2Cl_2]}](/tpl/images/1128/3654/65f33.png)
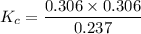
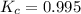
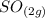 = 0.306 M
= 0.306 M

