Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1


Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
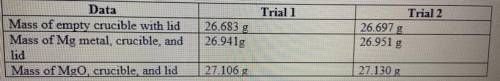
Magnesium is the limiting reactant in this experiment: Calculate the theoretical yield of MgO for ea...
Questions

Advanced Placement (AP), 28.11.2021 07:50

Mathematics, 28.11.2021 07:50

English, 28.11.2021 07:50

Mathematics, 28.11.2021 07:50

English, 28.11.2021 07:50

Chemistry, 28.11.2021 07:50

Mathematics, 28.11.2021 07:50





Chemistry, 28.11.2021 08:00

Mathematics, 28.11.2021 08:00


Biology, 28.11.2021 08:00


Mathematics, 28.11.2021 08:00

Computers and Technology, 28.11.2021 08:00

Mathematics, 28.11.2021 08:00

English, 28.11.2021 08:00