
Chemistry, 19.02.2021 09:40 lindseycagle00
Mole-mass problem:
Water decomposes to produce hydrogen gas and oxygen gas. How many moles of oxygen would be produced from 10.0 moles
water? 2 H2O → 2 H2 + O2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2


Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Mole-mass problem:
Water decomposes to produce hydrogen gas and oxygen gas. How many moles of oxyge...
Questions

Mathematics, 13.02.2022 01:20


Physics, 13.02.2022 01:20


Biology, 13.02.2022 01:20


Mathematics, 13.02.2022 01:20

Social Studies, 13.02.2022 01:20




English, 13.02.2022 01:20



Mathematics, 13.02.2022 01:20



Mathematics, 13.02.2022 01:20

Mathematics, 13.02.2022 01:20

History, 13.02.2022 01:20

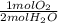 = 5.00 mol O₂
= 5.00 mol O₂

