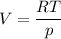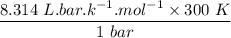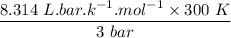Chemistry, 19.02.2021 17:00 bunbun2913
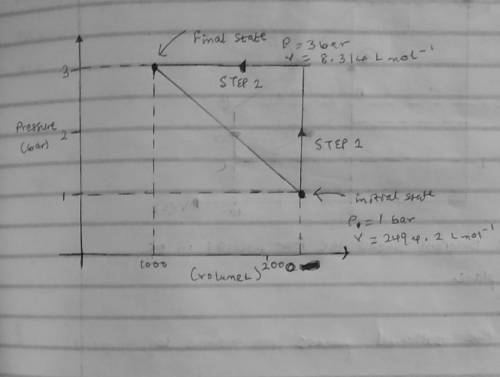
An ideal gas that is confined in piston-cylinder assembly (i. e., closed system) goes from an initial state of 1 bar at 300 K to a final state of 3 bar at 300 K by the following two-step process.
Process Path.
(Step 1) Heating at constant volume, and then
( Step 2) Cooling by holding the pressure constant.
Required:
a. Determine the initial and final molar mass.
b. Illustrate the two paths on a pressure-volume diagram. Clearly label the initial and final states, process steps and the direction of each step in the diagram.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1
You know the right answer?
An ideal gas that is confined in piston-cylinder assembly (i. e., closed system) goes from an initia...
Questions


English, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01





Mathematics, 21.10.2020 01:01




History, 21.10.2020 01:01



Mathematics, 21.10.2020 01:01