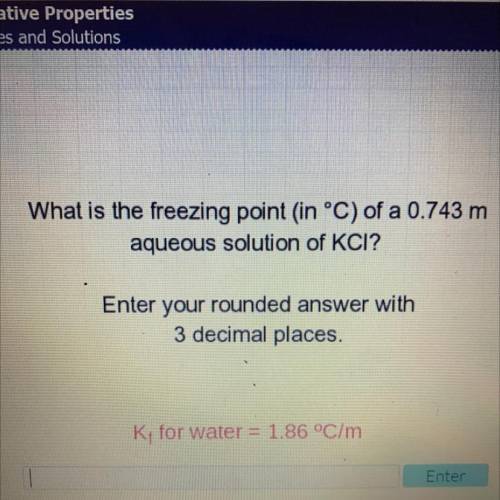
What is the freezing point in °C) of a 0.743 m
aqueous solution of KCI?
Enter your rounded an...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Questions


Mathematics, 30.03.2021 03:20

Spanish, 30.03.2021 03:20



Mathematics, 30.03.2021 03:20

Mathematics, 30.03.2021 03:20


Geography, 30.03.2021 03:20

Chemistry, 30.03.2021 03:20




Mathematics, 30.03.2021 03:20



History, 30.03.2021 03:20


History, 30.03.2021 03:20