
Chemistry, 19.02.2021 22:30 yaneiryx5476
1. Determine the percent composition for the following compound, given the empirical formula. (2 pts)
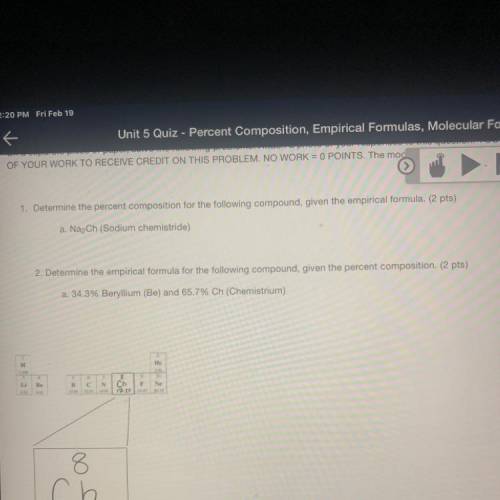
a. Na2Ch (Sodium chemistride)
2. Determine the empirical formula for the following compound, given the percent composition. (2 pts)
a. 34.3% Beryllium (Be) and 65.7% Ch (Chemistrium)
H
Не
Be
B
c С
N
Ch F Ne
17.250 30
8
ch
Chemistrium
17.25

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
1. Determine the percent composition for the following compound, given the empirical formula. (2 pts...
Questions

Mathematics, 09.02.2021 09:10



History, 09.02.2021 09:10

Mathematics, 09.02.2021 09:10

Mathematics, 09.02.2021 09:10



Mathematics, 09.02.2021 09:10

Mathematics, 09.02.2021 09:10

Mathematics, 09.02.2021 09:10



Mathematics, 09.02.2021 09:20

Mathematics, 09.02.2021 09:20


History, 09.02.2021 09:20



Mathematics, 09.02.2021 09:20



