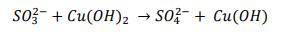Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution? a. 3.88 m, b. 1.03 m, c. 1.5 m, d. 15.5 m
Answers: 3

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

You know the right answer?
2. Consider an equation below:
a) Indicate the oxidation number of the elements that are reactants....
Questions


Biology, 27.06.2019 14:30


Health, 27.06.2019 14:30

Mathematics, 27.06.2019 14:30




History, 27.06.2019 14:30

Geography, 27.06.2019 14:30



Geography, 27.06.2019 14:30


Mathematics, 27.06.2019 14:30

English, 27.06.2019 14:30




Mathematics, 27.06.2019 14:30