
Chemistry, 21.02.2021 03:00 hopelesslylost13
1. 1 N2+ 3 H2→2 NH3 c. How many moles of nitrogen are needed to produce 12 moles of nitrogen trihydride?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
1. 1 N2+ 3 H2→2 NH3
c. How many moles of nitrogen are needed to produce 12 moles of nitrogen trihyd...
Questions

History, 28.08.2020 22:01



Arts, 28.08.2020 22:01






Mathematics, 28.08.2020 22:01

Mathematics, 28.08.2020 22:01

French, 28.08.2020 22:01

History, 28.08.2020 22:01



Mathematics, 28.08.2020 22:01




Mathematics, 28.08.2020 22:01

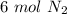
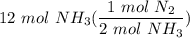 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 

