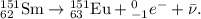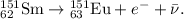Chemistry, 21.02.2021 04:40 joyceandtrey2781
Samarium – 151 is a beta emitter. Write an equation for this nuclear reaction and identify the product nucleus.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What are the concentrations of hydroxide and hydronium ions in a solution with a ph of 10.2?
Answers: 1


Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Samarium – 151 is a beta emitter. Write an equation for this nuclear reaction and identify the produ...
Questions

Mathematics, 08.12.2021 19:00




Biology, 08.12.2021 19:00



Biology, 08.12.2021 19:00


Computers and Technology, 08.12.2021 19:00


Geography, 08.12.2021 19:00


Mathematics, 08.12.2021 19:00

Mathematics, 08.12.2021 19:00


Mathematics, 08.12.2021 19:00

History, 08.12.2021 19:00


Computers and Technology, 08.12.2021 19:00

![\[_{Z}^{A}\textrm{X}\rightarrow _{Z\mp 1}^{A}\textrm{Y} + \beta ^\pm + \nu + \mathrm{Q}\]](/tpl/images/1134/0550/de5fd.png)
![\[_{Z}^{A}\textrm{X}\rightarrow _{Z+1}^{A}\textrm{Y} + \beta ^- + \bar{\nu} + \mathrm{Q}\]](/tpl/images/1134/0550/d7736.png)
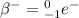 and
and 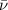 is an antineutrino.
is an antineutrino.