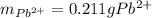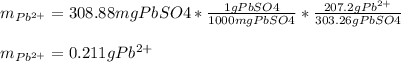Chemistry, 21.02.2021 09:20 hilzepesqtatiana
5 pos. 1. An excess of sodium sulfate was added to a 500. mL sample of polluted water. The
mass of lead (II) sulfate that precipitatcd was 308.88 mg. Determine the mass of lead that was in
the polluted water.
Na2SO4(aq) + Pb2+ (aq) → 2Na(aq) + PbSO4(s)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
5 pos. 1. An excess of sodium sulfate was added to a 500. mL sample of polluted water. The
mass of...
Questions


Mathematics, 02.03.2021 17:10

Mathematics, 02.03.2021 17:10


Mathematics, 02.03.2021 17:10

Mathematics, 02.03.2021 17:10





Social Studies, 02.03.2021 17:10

English, 02.03.2021 17:10


Biology, 02.03.2021 17:10

Spanish, 02.03.2021 17:10

Chemistry, 02.03.2021 17:10

Mathematics, 02.03.2021 17:10

Biology, 02.03.2021 17:10