
Chemistry, 21.02.2021 18:00 donaldwilliams31
Calculate the volume of oxygen liberated at s. t.p when 96500C of electricity is passed through aqueous solution of H2S04. (IF = 96500C, Volume at s. t.p = 22.4
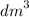

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1


Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
Calculate the volume of oxygen liberated at s. t.p when 96500C of electricity is passed through aque...
Questions

Business, 08.01.2021 05:30



Medicine, 08.01.2021 05:30

Social Studies, 08.01.2021 05:30



Mathematics, 08.01.2021 05:30

Mathematics, 08.01.2021 05:30


Mathematics, 08.01.2021 05:30

Chemistry, 08.01.2021 05:30


Mathematics, 08.01.2021 05:30


English, 08.01.2021 05:30







