Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 11:30
All of the following describe uses of nonrenewable energy sources except
Answers: 3

You know the right answer?
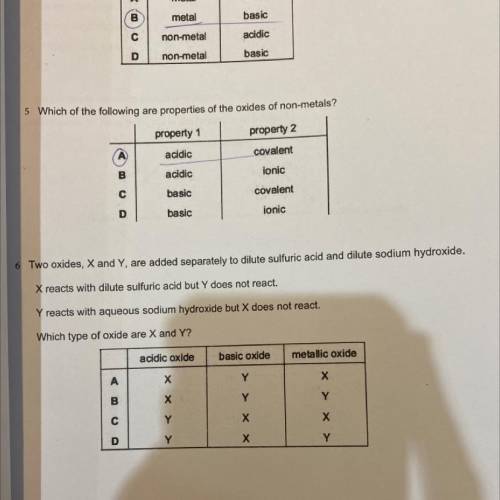
two oxides, X and Y are added seperately to dilute sulfuric acid and dilute sodium hydroxide. X reac...
Questions


Mathematics, 02.04.2020 20:08

Mathematics, 02.04.2020 20:08

Mathematics, 02.04.2020 20:08

English, 02.04.2020 20:08

Social Studies, 02.04.2020 20:08

Biology, 02.04.2020 20:08

Mathematics, 02.04.2020 20:08


Mathematics, 02.04.2020 20:08



Mathematics, 02.04.2020 20:08


Spanish, 02.04.2020 20:08

Physics, 02.04.2020 20:08

Mathematics, 02.04.2020 20:08

Mathematics, 02.04.2020 20:08