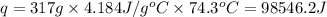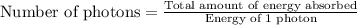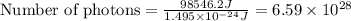Chemistry, 28.12.2019 18:31 stophendless9780
Acontainer with 0.317 l of water is placed into microwave and is then radiated with electromagnetic energy that has a wavelength of 13.3 cm. the temperature of the water then rose by 74.3 °c. calculate the number of photons that were absorbed by the water. assume water has a density of 1.00 g·ml–1 and its specific heat is 4.184 j·g–1·°c–1

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3


Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
Acontainer with 0.317 l of water is placed into microwave and is then radiated with electromagnetic...
Questions

Mathematics, 02.03.2021 01:40

Mathematics, 02.03.2021 01:40


History, 02.03.2021 01:40



Mathematics, 02.03.2021 01:40




English, 02.03.2021 01:40

Mathematics, 02.03.2021 01:40

Mathematics, 02.03.2021 01:40

Advanced Placement (AP), 02.03.2021 01:40






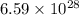
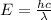
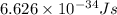
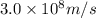
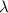 = wavelength of photon = 13.3 cm = 0.133 m (Conversion factor: 1 m = 100 cm )
= wavelength of photon = 13.3 cm = 0.133 m (Conversion factor: 1 m = 100 cm )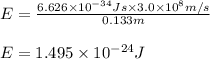
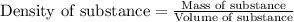
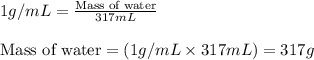
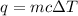
 = change in temperature = 74.3°C
= change in temperature = 74.3°C