
Chemistry, 22.02.2021 18:30 lovelybear2354
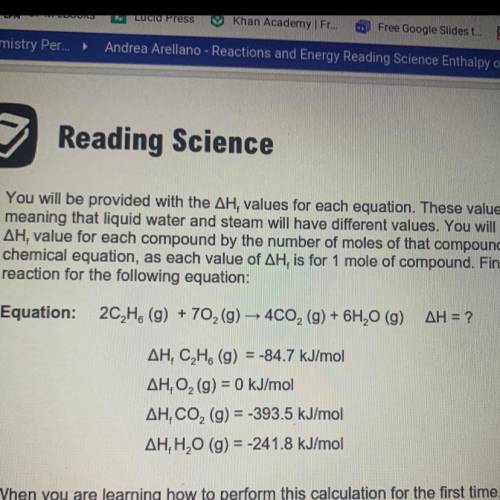
You will be provided with the AH values for each equation. These values are state dependent,
meaning that liquid water and steam will have different values. You will need to multiply the
AH, value for each compound by the number of moles of that compound in the balanced
chemical equation, as each value of AH, is for 1 mole of compound. Find the enthalpy of
reaction for the following equation:

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:40
Which of the following is a testable hypothesis? a. if i brush my teeth, i will get fewer cavities than if i don't brush my teeth. b. green toothpaste tastes better than blue toothpaste or red toothpaste. c. smart, careful, healthy people always brush their teeth. d. it's wrong to not brush your teeth before you have an important conversation with someone.
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

You know the right answer?
You will be provided with the AH values for each equation. These values are state dependent,
meanin...
Questions

Computers and Technology, 04.03.2021 20:00

Spanish, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00


Biology, 04.03.2021 20:00

Biology, 04.03.2021 20:00



Mathematics, 04.03.2021 20:00



Mathematics, 04.03.2021 20:00






History, 04.03.2021 20:00

Health, 04.03.2021 20:00

Mathematics, 04.03.2021 20:00



