
Chemistry, 23.02.2021 04:10 tammycute01
a rigid metal container has a volume of 20.0 L with nitrogen gas to a pressure of 197atm at 28.0 C. How many moles of gas are present in the container
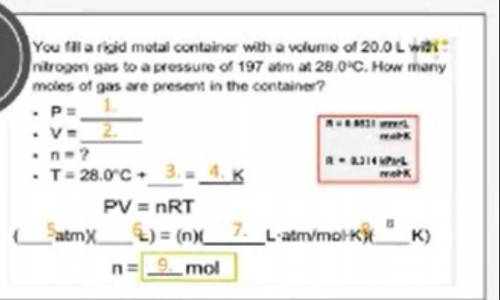

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1


Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
a rigid metal container has a volume of 20.0 L with nitrogen gas to a pressure of 197atm at 28.0 C....
Questions




Mathematics, 20.09.2020 09:01



Physics, 20.09.2020 09:01



Mathematics, 20.09.2020 09:01

Mathematics, 20.09.2020 09:01



History, 20.09.2020 09:01


Social Studies, 20.09.2020 09:01


English, 20.09.2020 09:01

Biology, 20.09.2020 09:01



