
Look at sample problem 19.10 in the 8th ed Silberberg book. Write the Ksp expression. Find the concentrations of the ions you need (in this case Ca2 and F-). Put those concentrations into the Ksp expression to calculate Q. Compare Q to K A common laboratory method for preparing a precipitate is to mix solutions containing the component ions. Does a precipitate form when 10. ml of 0.0010 M Ca(NO3)2 is mixed with 10. ml of 0.00010 M NaF

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 23.06.2019 10:30
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2

Chemistry, 23.06.2019 20:30
Due tomorrow write the chemical equation that has the equilibrium constant expression [listed in photo]
Answers: 2

Chemistry, 23.06.2019 22:00
2. a sample of table sugar (sucrose, c12h22o11) has a mass of 1.202 g. a. calculate the number of moles of c12h22o11 contained in the sample. show your work. b. calculate the moles of each element in c12h22o11, show your work c. calculate the number of atoms of each type in c12h22o11. show your work
Answers: 2
You know the right answer?
Look at sample problem 19.10 in the 8th ed Silberberg book. Write the Ksp expression. Find the conce...
Questions

Social Studies, 08.11.2020 22:10


Mathematics, 08.11.2020 22:10

Mathematics, 08.11.2020 22:10

Mathematics, 08.11.2020 22:10

Mathematics, 08.11.2020 22:10


Mathematics, 08.11.2020 22:10

Arts, 08.11.2020 22:10


Health, 08.11.2020 22:10



Computers and Technology, 08.11.2020 22:10

History, 08.11.2020 22:10


Social Studies, 08.11.2020 22:10

Mathematics, 08.11.2020 22:10


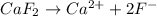
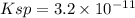
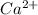 = 0.01 L × 0.0010 mol/L
= 0.01 L × 0.0010 mol/L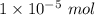
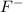 = 0.01 L × 0.00010 mol/L
= 0.01 L × 0.00010 mol/L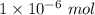
![[Ca^{2+}}] = \dfrac{1\times10^{-5} \ mol}{0.02 \ L} \\ \\ \\ \[[Ca^{2+}}] = 0.0005 \ mol/L](/tpl/images/1138/9149/5dc3a.png)
![[F^{-}] = \dfrac{(1\times 10^{-6} \ mol)}{0.02 \ L}](/tpl/images/1138/9149/ecdb2.png)
![[F^{-}] = 5 \times 10^{-5} \ mol/L](/tpl/images/1138/9149/20d72.png)
![Q = [Ca^{2+}][F^-]^2 \\ \\ Q = 0.0005 \times (5\times 10^{-5})^2 \\ \\ Q = 1.25 \times 10^{-12}](/tpl/images/1138/9149/52d7c.png)


