
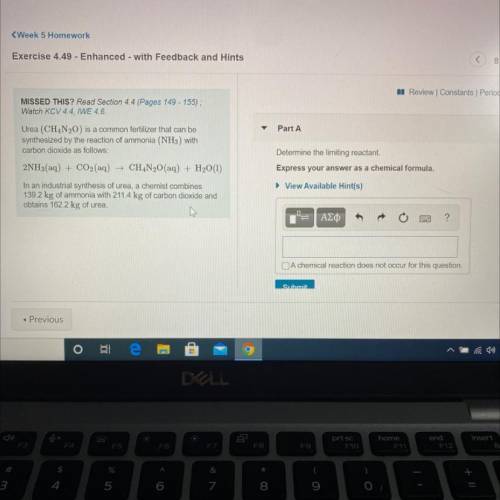
Urea (CH4N20) is a common fertilizer that can be
synthesized by the reaction of ammonia (NH3) with
carbon dioxide as follows:
Determine the limiting reactant.
Express your answer as a chemical formula.
2NH3(aq) + CO2(aq) + CH4N20(aq) + H2O(1)
In an industrial synthesis of urea, a chemist combines
139.2 kg of ammonia with 211.4 kg of carbon dioxide and
obtains 162.2 kg of urea.
Determine the limiting reactant

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:40
What kind of ion is contained in salts that produce an acidic solution? a positive ion that attracts a proton from water a positive ion that releases a proton to water a negative ion that attracts a proton from water a negative ion that releases a proton to water
Answers: 1

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
Urea (CH4N20) is a common fertilizer that can be
synthesized by the reaction of ammonia (NH3) with...
Questions

Mathematics, 15.11.2020 07:50

Mathematics, 15.11.2020 07:50

History, 15.11.2020 07:50


History, 15.11.2020 07:50

Mathematics, 15.11.2020 07:50


Mathematics, 15.11.2020 07:50



English, 15.11.2020 07:50

Mathematics, 15.11.2020 07:50

Biology, 15.11.2020 08:00


World Languages, 15.11.2020 08:00


Mathematics, 15.11.2020 08:00



English, 15.11.2020 08:00




