compute the theoretical yield of the product (in

Chemistry, 23.02.2021 09:10 marissagirl9893
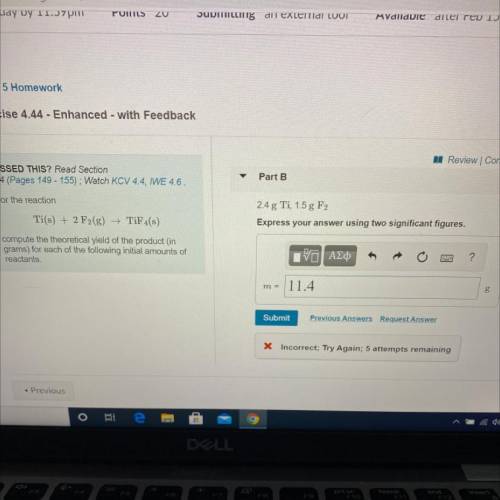
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
grams) for each of the following initial amounts of
reactants.
2.4 g Ti, 1.5 g F2
Express your answer using two significant figures.
please help! will give brainliest.

Answers: 3
Another question on Chemistry


Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1
You know the right answer?
For the reaction
Ti(s) + 2 F2(g) →TiF4(s)
compute the theoretical yield of the product (in
compute the theoretical yield of the product (in
Questions

Mathematics, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00

Chemistry, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00


Mathematics, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00

History, 20.01.2021 07:00

History, 20.01.2021 07:00

Chemistry, 20.01.2021 07:00

Mathematics, 20.01.2021 07:00


Biology, 20.01.2021 07:00

English, 20.01.2021 07:00


History, 20.01.2021 07:00

Social Studies, 20.01.2021 07:00



