
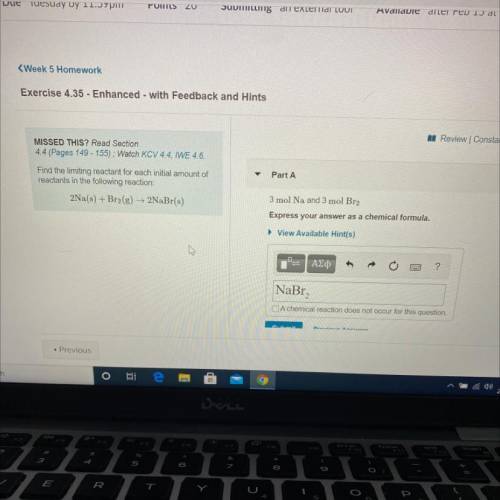
Find the limiting reactant for each initial amount of
reactants in the following reaction: 2Na(s) + Br2(g) + 2NaBr(s)
Part A: 3 mol Na and 3 mol Br2
Part B: 1.6 mol Na and 1.2 mol Br2
Part C: 2.5 mol Na and 1 mol Br2
Part D: 12.9 mol Na and 6.9 mol Br2
Express your answers as a chemical formula.
Please help! will give brainliest

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Find the limiting reactant for each initial amount of
reactants in the following reaction: 2Na(s) +...
Questions







Mathematics, 31.05.2020 03:04




Mathematics, 31.05.2020 03:04





Mathematics, 31.05.2020 03:04






