
Chemistry, 23.02.2021 09:20 keilajohnson810
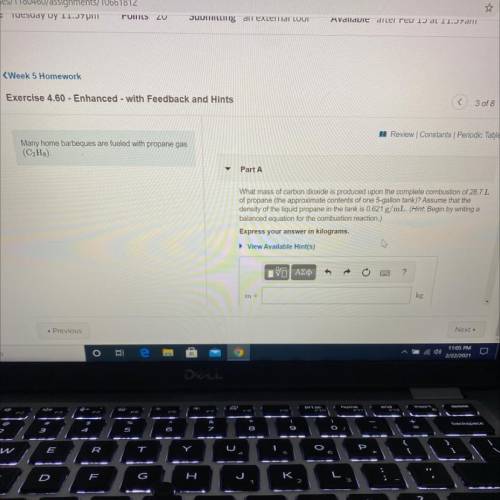
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is produced upon the complete combustion of 28.7L
of propane (the approximate contents of one 5-gallon tank)? Assume that the
density of the liquid propane in the tank is 0.621 g/mL.
Express your answer in kilograms.
Will give brainliest!

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Someone, part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 1

Chemistry, 21.06.2019 16:30
How many atoms of oxygen are contained in 160 grams of n2o3
Answers: 2

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3
You know the right answer?
Many home barbeques are fueled with propane gas
(C3H8)
Part A: What mass of carbon dioxide is...
Part A: What mass of carbon dioxide is...
Questions


Mathematics, 25.08.2020 16:01

Spanish, 25.08.2020 16:01

Engineering, 25.08.2020 16:01

History, 25.08.2020 16:01


Chemistry, 25.08.2020 16:01

History, 25.08.2020 16:01




Biology, 25.08.2020 16:01

Mathematics, 25.08.2020 16:01

Mathematics, 25.08.2020 16:01

Spanish, 25.08.2020 16:01


Business, 25.08.2020 16:01

Chemistry, 25.08.2020 16:01


Mathematics, 25.08.2020 16:01



