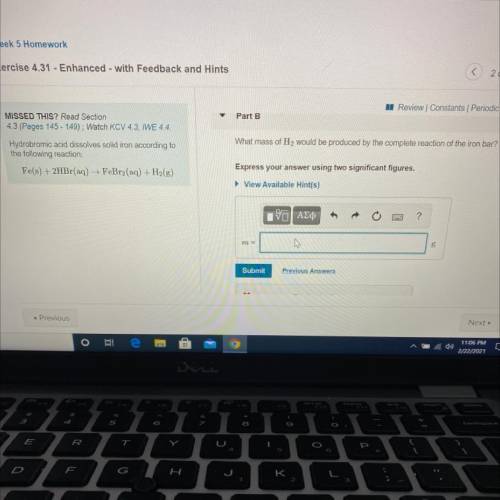
Hydrobromic acid dissolves solid iron according to
the following reaction:
Fe(s) + 2HBr(aq) →...

Chemistry, 23.02.2021 09:10 suttonfae336
Hydrobromic acid dissolves solid iron according to
the following reaction:
Fe(s) + 2HBr(aq) → FeBr2(aq) + H2(g)
What mass of H2 would be produced by the complete reaction of the iron bar?
Express your answer using two significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

You know the right answer?
Questions


Mathematics, 16.01.2020 21:31


Biology, 16.01.2020 21:31

Mathematics, 16.01.2020 21:31




History, 16.01.2020 21:31

Mathematics, 16.01.2020 21:31


Geography, 16.01.2020 21:31

English, 16.01.2020 21:31


Physics, 16.01.2020 21:31

Mathematics, 16.01.2020 21:31



History, 16.01.2020 21:31



